Rheo-Cooling applied to
Plastics.
As the primary use of a mold is to shape material into an object,
not to cool it, conventional molds are not designed to apply specific cooling
treatments to materials, although they are implicitly used as heat transfer
agents to solidify the material. When Rheo-Cooling
development is aimed at implementing specific cooling treatments and the
material cooled is a plastic in a mold, this implementation has been called
“Rheomolding”.
Rheomolding based processes use rheology principles to vary the
value of physical transitions (Tg, Tm) as the material temperature decreases to cross them at any given
speed. Take a rubber, for instance: its modulus depends on the value of (T-Tg) where T is the temperature and Tg is the glass transition temperature. Tg is itself function of pressure and of the frequency and amplitude
of a vibration if such a mechanical force is exerted on it (shear or pressure
vibration).
The figure below shows the pressure dependence of Tg for Polystyrene. Tg varies by 32 oC/Kbar. This means that if one applies a
pressure variation of 1 Kbar per sec, for instance, one obtains a Rheo-Cooling
rate of 32 oC/ sec, i.e. 1920 oC/min!. This is not
achievable by conduction, especially for a bulk material, yet Rheo-Cooling
gives you such possibilities (see the previous blog: Rheo-Cooling 1 l of water
in less than 5 sec)!
Fig. 1 shows that the rate
of cooling influences the state of the
glass once the Tg is
crossed. We can measure the specific volume at different temperatures while the
material cools: the break and the change of slope corresponds to the Tg; Fig. 1 shows that if the
material is quenched, its Tg is higher and the glass
produced is in a state of non-equilibrium which is more pronounced.
In fact one can demonstrate
that the material cooled faster is in a different state of non-equilibrium by
studying the DSC trace obtained by heating a piece of it after it has cooled to
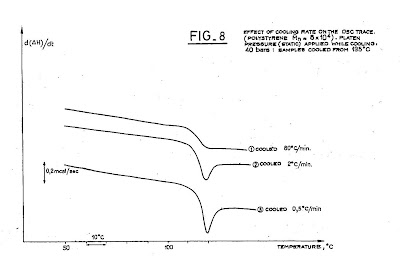
room temperature. This is shown in the following figure (“Figure 8”).
One sees that the “fast cooling”, at 80 oC/min produces
on heating a characteristic DSC trace of quenched glasses: there is only a drop
of the baseline occurring at Tg, no peak visible. For the 2oC/min cooling rate, a peak
is visible, indicating an overshoot of the heat capacity before the liquid
state is reached. This peak is more pronounced for the even smaller cooling
rate of 0.5 oC/min.
OK, we have in hand a nice way to know if the glass was slowly
cooled or “quenched” during its solidification: we can run a DSC scan on it and determine the
size of the peak at Tg. This can be very
useful to characterize the glasses formed by Rheo-Cooling.
What other physical parameter can we think of to alter the value
of phase transitions, besides pressure?
The Ta transition is the mechanical representation of Tg. What this figure says is that Tg is dependent on the value of the frequency when the material is
oscillated, like it is dependent on pressure. The Frequency map shows that the
value of Tg varies by 49 oC when
the frequency varies from 10-1.5 Hz (the point corresponding to 1000/T = 2.725) to 105
Hz (the point corresponding to 1000/T = 2.4). In other words, one can sweep
frequency to increase or decrease at a given rate and this will correspond to
Rheo-Cooling or Rheo-Heating effects. For instance, one can choose to vary
linearly the logarithmic of the frequency to simulate cooling rates and produce
various types of non-equilibrium states for the glass formed under such
conditions.
Frequency is swept from low to high value as temperature
decreases, raising the value of Tg at any chosen rate. The amplitude of the oscillation is also
increasing during the same time. These oscillations are continued until the
material temperature is reduced to a level where frequency effects are no
longer required to maintain product integrity (~ 20 oC below Tg). In the video we hear the
frequency increase from 1 Hz to 3000 Hz.
The sample is a Polystyrene disk confined in a mold at room temperature, heated
to an initial temperature where it is a viscoelastic rubber, pressurized in the
mold to an initial pressure which can vary between 500 and 10,000 PSI, the
pressure being capable of oscillation of 0 to 100% in excursion. The apparatus
has cooling channels and heating cartridges disposed in the mold to control the
rate of cooling by passage of refrigerant fluids, such as water, a mix of
glycerol and water, or compressed air. The lower mold half is attached to a
vibrating table which imposes the pressure excursion to the plastic disk
through an accelerometer. The upper mold half is fixed and connected to a
pressure cell. A pneumatic air piston, shaped like a flat inflatable disk,
transmits the average hydrostatic force while the electromagnetic shaking table
exerts the pressure excursion.
We see in the video the different elements composing the
Rheomolding apparatus set up: watch the technician take the machine apart, once
the Rheo-cooling experiment is done, in order to separate the two mold halves
and extract the treated polystyrene disk. This treated sample is submitted to a series
of tests to determine the difference in properties between references (purely
static runs) and “Rheomolded” conditions. We characterized the thermal,
mechanical and dielectric properties of these materials.
We run DSC tests for the treated samples to reveal what type of non-equilibrium states were achieved by Rheo-cooling or Rheo-heating while the glass was being cooled by conduction. The figure below (“Fig. 7”) tells the story for two treatments which are, each time, compared to a reference sample obtained under the same cooling conditions , except without any frequency sweep in the mold ( “no vibration”).
We run DSC tests for the treated samples to reveal what type of non-equilibrium states were achieved by Rheo-cooling or Rheo-heating while the glass was being cooled by conduction. The figure below (“Fig. 7”) tells the story for two treatments which are, each time, compared to a reference sample obtained under the same cooling conditions , except without any frequency sweep in the mold ( “no vibration”).
Treated sample 1:
The REFERENCE curve for treated sample 1 is the 2nd curve
from the top. It shows a typical drop off of the Heat Flow baseline at Tg, characteristic of a fast cooled sample (80 oC/min).
The top DSC trace, however, corresponding to the TREATED sample, is very
strange: the DSC trace displays a strong peak at Tg, a characteristic of a very slowly cooled specimen, but,
noticeably, the baselines for the melt and for the glass are the same, unlike for
slowly cooled samples (see above). This is as if there was no longer a
difference in the Heat Capacity below and above Tg: DCp(@ Tg)=0!
Treated sample 2 (lower two traces):
The REFERENCE is, again, typical of a fast cooled sample: no peak
at Tg. The treated sample is very
different from the reference sample cooled identically but with no vibration
during cooling. The endothermal characteristics at Tg are more complex for the treated than for the untreated sample.
In particular, the endotherm at Tg is broader for the treated sample, extending over 40 oC
range above Tg. Then, the baseline seems to be
shifted upward by an exothermic wave, in a way similar to what happened to the
treated sample 2. At the end, up in the
high melting region, the heat capacity seems to extrapolate from the solid
region, similarly to what was seen for treatment
1.
In conclusion, it appears that the sample which has been
Rheo-Cooled had thermal characteristics at room temperature (after the
treatment) which could be extrapolated from the liquid region where it is known
that the polymer is at equilibrium. This would mean that the treated sample
behaved below Tg like a sample which had reached equilibrium, i.e. like a specimen
which has been extensively annealed.
The other possibility that comes to mind is to challenge the fact
that the liquid region, above Tg, is at equilibrium; in other words, Rheo-Cooling can create
non-equilibrium liquid states.
It took me to build another machine, the Rheo-Fluidizer, the subject of recent publications, to prove that the liquid
state could, indeed, be brought out of equilibrium with respect to its state of
entanglement.
This brings us to the Split-Dual-Phase model of entanglement, which
I have discussed already in previous blogs and lectures.
Something is clear to this author: melt manipulation such as
Rheo-Cooling and/or Rheo-Fluidification, or any combination thereof, gives us formidable
new tools to prepare new plastics; and, for a change, this is not based on what chemists can do: synthesize millions of new molecules and their blends; but rather it is because a new
understanding of the physics of the interactions between the macromolecules
opens a wide new door to the way they can be processed: the age of Smart Processing can proceed!
PS
I will provide in a separate seminar the processing details for
the experiments described in this blog.